The question is no longer whether or not Medicare will do it but when and how. The initiation of a National Coverage Decision (NCD) for Dendreon’s Provenge® has triggered a lot of discussion about the cost of cancer drugs, and whether they are worth the benefit. But, is the Provenge NCD an indicator of things to come? It is being speculated that Medicare will cover a very defined patient population in an attempt to limit the off-label use and cost of Provenge. But the Social Security Act, which defines what Medicare will cover, requires coverage of all “reasonable and necessary” treatments and contains no provision for pricing considerations. This post will review why Medicare must begin to control cancer drug costs, the restrictions they have been under thus far, how they are controlling costs today and how they will most likely control them in the future.
In the US, manufacturers have been able to freely price therapeutics with little concern that a premium price could affect demand. Although Medicare is required to pay for cancer drugs regardless of price, third-party payers are also mandated by many states to cover cancer treatments and they usually follow Medicare’s lead regarding coverage. This broad coverage of cancer drugs coupled with a lack of price regulation has created a situation where the risk that a drug company could price a product too high has, thus far, been minimal. The major concern of drug company management is the negative P.R. that a company and the industry could receive for setting prices too high and running the risk of reaching a “tipping point”—the point at which the price of a drug is so high that it activates Congress to make changes (on behalf of the public) to the Social Security Act to control drug pricing.
Oncology drug prices have been increasing rapidly and threaten to bankrupt the Medicare Trust Funds. As illustrated in Figure 1, the monthly cost of oncology drugs at approval is on the rise.
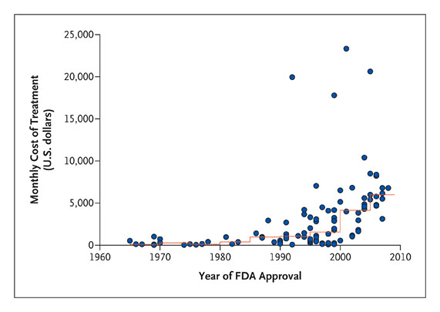 Figure 1. The Rising Price of New Oncology Drugs. Source: Peter B. Bach, M.D., M.A.P.P. N Engl J Med. 2009;360:626-633.
Figure 1. The Rising Price of New Oncology Drugs. Source: Peter B. Bach, M.D., M.A.P.P. N Engl J Med. 2009;360:626-633.
In addition, Medicare Part B spending has grown from $3 billion in 1997 to $11 billion in 2004—a 267% increase—and, according to Peter Bach, MD, in a NEJM article, cancer costs have risen faster than many parts of healthcare. Insolvency of the Medicare Trust Funds and the rapidly increasing share of the Federal budget assigned to Medicare is an issue that concerns both Republicans and Democrats.
Today, Medicare and the governments’ ability to control cancer drug costs has been limited by the laws, regulations and court rulings described in Table 2. So how does Medicare manage drug costs?
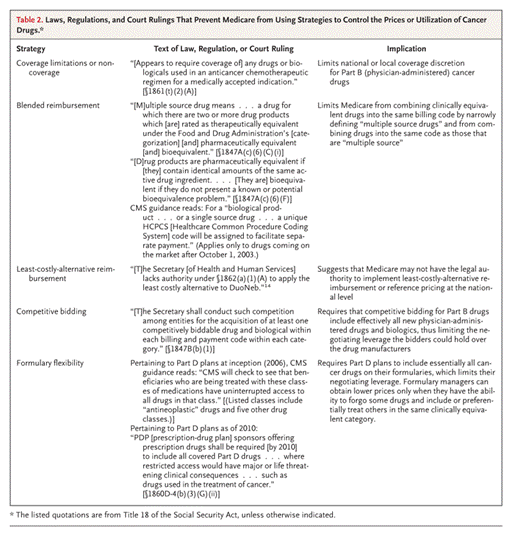 Source: Peter B. Bach, M.D., M.A.P.P. N Engl J Med 2009; 360:626-633.
Source: Peter B. Bach, M.D., M.A.P.P. N Engl J Med 2009; 360:626-633.
One mechanism they have is using the National Coverage Decision to limit utilization by strictly defining the patient population eligible for coverage as is anticipated with the Provenge NCD. Another mechanism is using the Least Costly Alternative (LCA) rule. Medicare has defined “reasonable and necessary” to encompass cost considerations when products are classified as “clinically interchangeable”, they’ve been able to set a reimbursement rate that is equal to the weighted average price of the “interchangeable” products or the price of the least expensive product.
Medicare has been able to effectively use the LCA to manage the price of LHRH agonists for prostate cancer for many years. Though there have been some legal challenges to the use of LCA I expect Medicare will increase its use of it.
In an attempt to control their budget, I believe that Medicare will become more aggressive in defining products as clinically interchangeable in order to implement LCA pricing. The government has set aside $1.1 billion for comparative effectiveness research towards this effort. It appears that this research will provide the impetus for broadening the interchangeability language in the Social Security Act and give Medicare the ability to reimburse at the LCA rate for many more products regardless of their composition, class, or mechanism of action.
More will be revealed as the details of the comparative effectiveness studies to be conducted emerge, but there is no doubt that cost controls will eventually impact oncology. Government agencies as well as taxpayers are at their tipping point of what can be considered affordable healthcare, and the NCD for Provenge is the first indication of things to come.